White Adipose Tissue Assay analysis can be effective - just do it with us!
application for white adipose tissue assay analysis
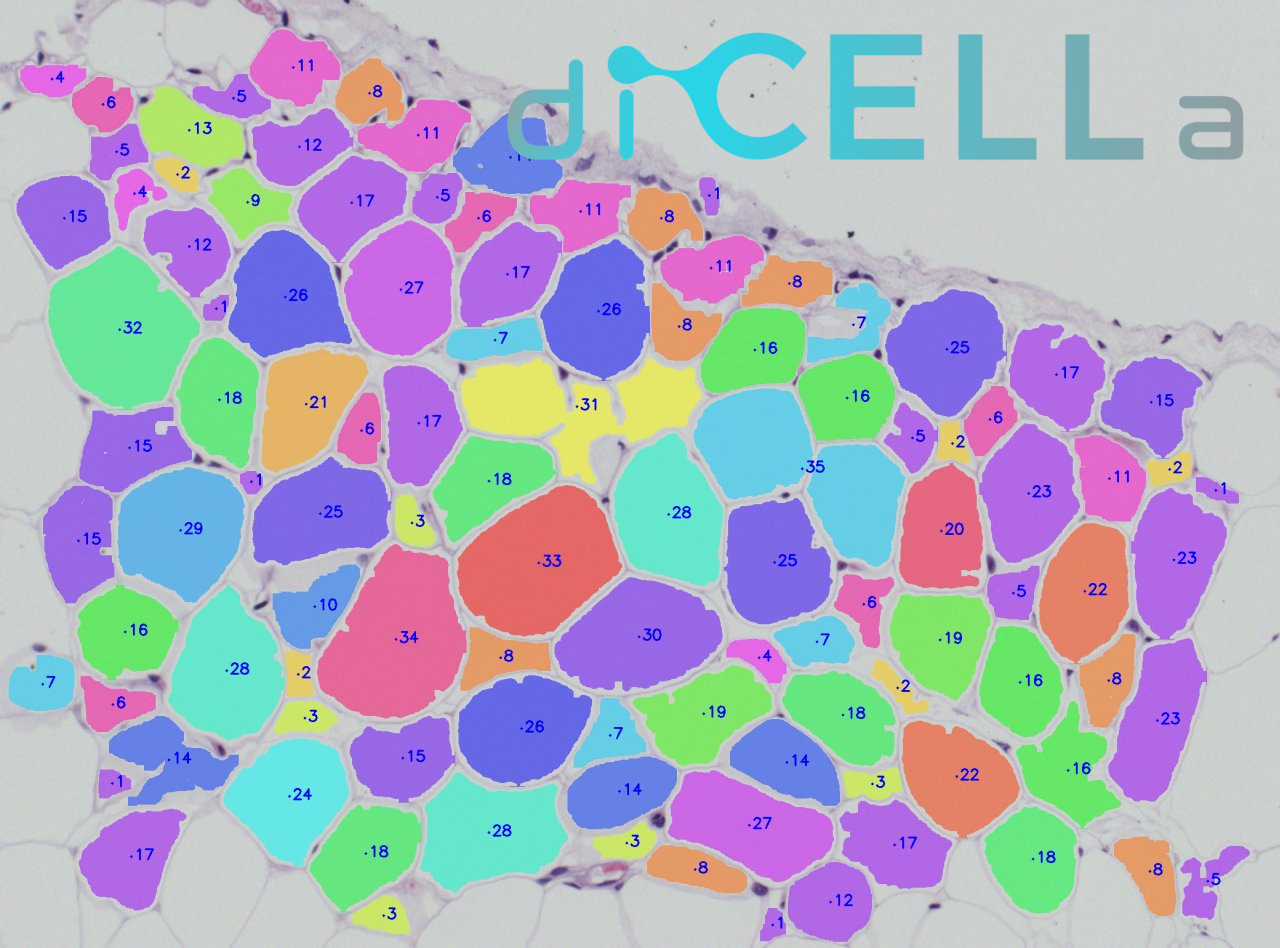
diCELLa White ADIPOSE Tissue is an application that allows you to quickly analyze results of your experiment. Given only unmodified images our algorithm detects adipose tissue cells and provides you with a number of useful information.
The algorithm:
- allows to analyze a wide range of images coming from different imaging devices,
- works on cells stained with hematoxylin and eosin solution,
- is suitable for experiments exploring increase in the size and the number of adipocytes under different conditions.
Our solution is fast, automatic, reliable
How to use it?
To use the diCELLa White ADIPOSE Tissue you need to upload a number of images (max. size 7MB per image) in any image format. The software works on images taken using light microscope.
Results are available to download in txt or pdf format just few seconds after running the experiment in Image Service. They consist of cell amount, mean cell area, along with other statistical information. Output image is also available, with each adipose tissue cell marked in different color.
Create an account on our Image Service platform, buy dicellons and exchange them to upload your images.
If you want to use your results in a scientific paper or during a speech at a scientific conference, contact us to get a discount!
Scientific Background
Adipose tissue is an multifunctional organ which is associated with many physiological functions. It plays a protective role, stores fat and is the biggest endocrine organ. These tissue is physiologically and morphologically differentiated. Adipocytes which built adipose tissue might be divided into two main groups: white (which build white adipose tissue-WAT) and brown (which build brown adipose tissue-BAT). Their morphology is differentiated due to genetic, metabolic and environmental reasons [1,2,3].
The main role of white adipose tissue is storage of triglycerides during increased energy supply and using them during periods with increased energy expenditure. The percentage of this tissue is highly variable: from 3% in athletes to even 70% in obese people [4]. In humans these tissue is produced during fetal life (14-24 weeks of pregnancy) [5,6], whereas in rats and mice it is produced postnatally [7,8].
The morphological and functional changes in WAT occurs with obesity. The surplus energy stored in the form of triacylglycerols during chronic positive energy imbalance results in changed of adipocytes size (hypertrophy) and in number (hyperplasia) [9]. Adipose hypertrophy results from a comparative increase in lipid deposition against to lipolysis [10,11]. The hyperplasia of adipocytes results as effect of adipogenesis. It is process of preadipocytes (adipocyte precursors) compensation by dividing and differentitation into adipocytes. It occurs when demand for lipid storage transcends the existent adipocytes [12,13,14,15].
WAT increasing is associated with obesity, Cushing’s syndrome, hypogonadism. Waste of adipose tissue occurs in many pathological situations, like cachexia, certain parasitic infection and HIV infection [16,17,18,19,20]. Measuring the size and number of adipocytes in deposition, development and remodeling of WAT is relevant in phenotype characterization of a given adipose tissue depot.
Actually there is a few popular methods to quantitative histomorphometry of these tissue. Most of them require the enzymatic disruption of tissue to isolate the individual population of adipocytes, which might be stained and analyzed by coulter counter or flow cytometry [21,22,23]. The main disadvantage of these methods is destruction or elimination of some part of adipocytes after enzymatic digestion, centrifugion or mesh separation. The main drawback of flow cytometry is separation of adipocytes by size as a result of analysis of internal lipid accumulation [24].
In other technologies scientists might use different lipophilic fluorophores which bound to the lipids in adipocytes. Confocal analysis of this results is expensive and tedious (1000 adipocytes is needed for accuracy) [25].
Another substances which are useful to adipocyte staining might be toxic and promote adipocyte swelling (e.g. osmium tetroxide which is used in microcomputed tomography to visualize narrow adipocytes) [22,26].
The most popular staining of adipocytes is hematoxylin and eosin solution. Dicella proposed semiautomated software to analyze adipocytes size and number. These software is easy to use and rapid.
References:
[1] E. Murawska-Ciałowicz, Tkanka tłuszczowa – charakterystyka morfologiczna i biochemiczna różnych depozytów, Postepy Hig Med Dosw (online), 2017; 71: 466-484
[2] Cinti S.: The adipose organ. Prostaglandins Leukot. Essent. Fatty Acids, 2005; 73: 9-15
[3] Giordano A., Smorlesi A., Frontini A., Barbatelli G., Cinti S.: White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur. J. Endocrinol., 2014; 170: R159-R171
[4] Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obesity Reviews. 2001;2(4):239–254.
[5] Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annual Review of Nutrition. 1992;12:207–233
[6] Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Human Development. 1984;10(1–2):1–11
[7] Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, et al. The spatiotemporal development of adipose tissue. Development. 2011;138(22):5027–5037
[8] Pouteau E, Turner S, Aprikian O, Hellerstein M, Moser M, Darimont C, et al. Time course and dynamics of adipose tissue development in obese and lean Zucker rat pups. International Journal of Obesity. 2008;32(4):648–657
[9] Poretsky L, Ebooks Corporation Principles of Diabetes Mellitus. 2010 Retrieved fromhttp://www.msvu.ca:2048/login?url=http://www.msvu.eblib.com/EBLWeb/patron?target=patron&extendedid=P_511708_0&
[10] Kaartinen JM, LaNoue KF, Martin LF, Vikman HL, Ohisalo JJ. Beta-adrenergic responsiveness of adenylate cyclase in human adipocyte plasma membranes in obesity and after massive weight reduction. Metabolism. 1995;44(10):1288–1292. PII: 0026-0495(95)90031-4.
[11] Reynisdottir S, Ellerfeldt K, Wahrenberg H, Lithell H, Arner P. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. The Journal of Clinical Investigation. 1994;93(6):2590–2599.
[12] Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: Going back to the future. Journal of Lipid Research. 2012a;53(2):227–246.
[13] Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells: The great WAT hope. Trends in Endocrinology and Metabolism. 2012b;23(6):270–277.
[14] de Ferranti S, Mozaffarian D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clinical Chemistry. 2008;54(6):945–955.
[15] Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: A new model of obesity. The American Journal of Physiology. 1978;235(3):E279–286.
[16] Desruisseaux MS, Nagajyothi, Trujillo ME, Tanowitz HB, Scherer PE. Adipocyte, adipose tissue, and infectious disease. Infection and Immunity. 2007;75(3):1066–1078.
[17] Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochimica et Biophysica Acta. 2013pii: S0925–4439(13)00191–9
[18] Santosa S, Jensen MD. Effects of male hypogonadism on regional adipose tissue fatty acid storage and lipogenic proteins. PLoS One. 2012;7(2):e31473
[19] Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667–684.
[20] Tisdale MJ. Biology of cachexia. Journal of the National Cancer Institute. 1997;89(23):1763–1773.
[21] Bradshaw AD, Graves DC, Motamed K, Sage EH. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):6045–6050.
[22] Hirsch J, Gallian E. Methods for the determination of adipose cell size in man and animals. Journal of Lipid Research. 1968;9(1):110–119.
[23] Maroni BJ, Haesemeyer R, Wilson LK, DiGirolamo M. Electronic determination of size and number in isolated unfixed adipocyte populations. Journal of Lipid Research. 1990;31(9):1703–1709.
[24] Lee YH, Chen SY, Wiesner RJ, Huang YF. Simple flow cytometric method used to assess lipid accumulation in fat cells. Journal of Lipid Research. 2004;45(6):1162–1167.
[25] Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56(6):1517–1526.
[26] Sebastian D. Parlee, et al., Quantifying Size and Number of Adipocytes in Adipose Tissue, Methods Enzymol. Author manuscript; available in PMC 2015 Jan 1
